|
|
 |
 |
 |
 |
 |
 |
Small molecules that activate or suppress biological events have become powerful tools to understand complex cellular processes. Importantly, they can be used as therapeutic agents to treat various diseases. Research goal of Center for Biofunctional Molecules includes 1) development of biofunctional molecules that regulate various biological processes, 2) elucidation of their mechanisms of action and 3) their applications to treating diseases (“Chemical Biology”). To achieve this goal, Center for Biofunctional Molecules consists of organic and biological researchers who collaborate with each other closely to promote a successful outcome. Specifically, organic researchers synthesize various small molecules, carbohydrates and peptides as well as create drug delivery systems. In addition, they construct microarrays containing a number of glycans and peptides to rapidly analyze biomolecular recognition events. Biological researchers carry out high-throughput screening to identify small molecules that regulate numerous biological functions, in particular cell death and differentiation, and study their mechanisms of action in cells or animal model. Ultimately, we hope to provide diverse chemical tools that are useful for biological research and develop novel therapeutic agents.
|

|
| Glycans in cells and organisms play roles in a wide range of physiological processes, such as cell communication, cell adhesion and trafficking, through interactions with glycan-binding proteins. Importantly, these biomolecular interactions are also implicated in various pathological processes. On this basis, elucidation of glycan-associated binding events and their consequences is highly important and interesting in basic biological research and biomedical applications. To rapidly assess glycan-associated recognition events, we have constructed several types of glycan microarrays containing various glycans. We have demonstrated that this microarray technology is ideally suited to study glycan-mediated binding events because they enable multiple parallel analyses of glycan-protein interactions using only small amounts of glycan samples. Nowadays, this microarray technology has become a leading edge tool in studies aimed at elucidating roles played by glycans and glycan binding proteins in biological systems. To date, we have applied this microarray technology for rapid analysis of the glycan binding properties of lectins and antibodies, the quantitative measurements of glycan-protein interactions, detection of cells and pathogens, fast assessment of substrate specificities of glycosyltransferases, and rapid identification of functional glycans that elicit cell surface lectin-mediated cellular responses. |
 |
|
| We are also interested in preparing glycoclusters that efficiently detect mammalian cells and pathogens expressing lectins on their surfaces. For example, glycan-conjugated, fluorescent and magnetic nanoparticles have been constructed and employed to detect Helicobacter pylori that is known to cause chronic gastritis, which may lead to peptic ulcer disease and gastric cancer. These dual-modal glyconanoparticles have been also used to enrich the pathogen and to block adhesion of H. pylori to mammalian cells. In addition, peptide-based glycoclusters have been prepared to probe mammalian cell-surface carbohydrate binding proteins. |
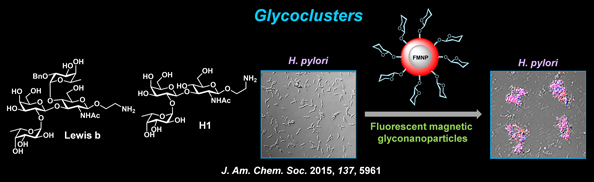 |
|

|
| Damaged tissues are normally regenerated by differentiation of cells. However, certain types of cells, such as neurons and cardiomyocytes, are not well regenerated under diseased conditions. In this case, cell therapy (or cell transplantation) is needed to regain function. Over a last decade, various cells have been generated from multipotent and pluripotent stem cells by introducing specific genes into cells. However, insertion of exogenous genetic materials into the host genome may be associated with unpredictable side effects. To avoid this issue, we have made an attempt to identify small molecules that convert somatic cells into specific cells (small molecule-based cellular alchemy). For example, we identified the first small molecule named neurodazine (Nz) that differentiates human/mouse skeletal muscle cells into neurogenic cells by using high-throughput screening approach. This substance was also found to have neurogenesis inducing activity in mouse embryonic carcinoma P19 cells, fibroblasts and neuroblastoma cells. Furthermore, we have shown that a sirtuin-1 selective inhibitor differentiates P19 cells into neurons with electrophysiological properties. Our goal of this research field is to uncover various small molecules that promote differentiation of somatic cells into a certain type of cells. |
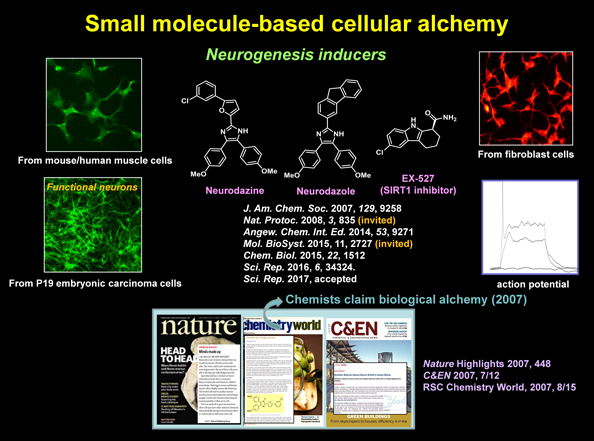 |
|

|
| Apoptosis (or programmed cell death) is an important biological process to maintain tissue homeostasis and to remove unwanted or damaged cells. Tumour cells are known to be resistant to apoptotic cell death via multiple anti-apoptotic processes. Thus, it is of great importance to discover potent apoptosis inducers that can be utilized as anticancer agents. Autophagy is a self-eating process that contributes to cell survival under starvation conditions. During autophagy, subcellular organelles and other cell components are sequestered in double-membrane vesicles termed autophagosomes, and they are delivered to lysosomes for digestion and recycling. It is known that levels of autophagy in cancer cells are generally higher than those in normal cells, leading to suggestions that autophagy-disrupting agents may be potentially useful in treatment of cancers. We are interested in developing potent apoptosis and/or autophagy regulating small molecules. For instance, we have developed a small molecule named apoptozole (Az) that induces apoptosis in cancer cells by using high-throughput screening of small molecule libraries. We found that this molecule has apoptosis inducing activity by inhibiting Hsp70 proteins. We are also interested in uncovering novel autophagy regulating small molecules by using cell- and protein-based high-throughput methods. |
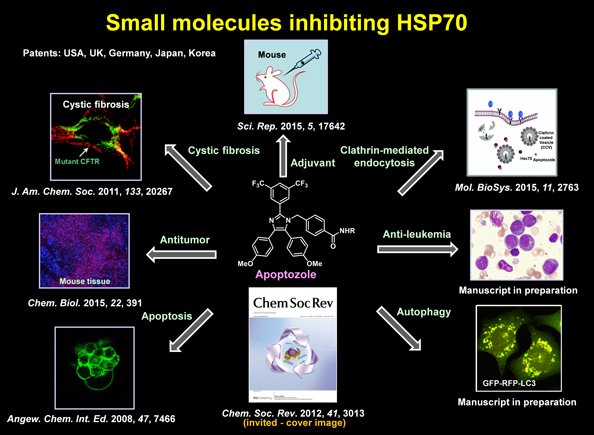 |
|

|
| Maintenance of ion homeostasis of cells is essential for sustaining life processes, including proliferation, differentiation and apoptosis. Various cancer cells exhibit different patterns of ion channels, resulting in the dysregulation of ion concentrations and fluxes. Synthetic or semi-synthetic ion carriers or transporters that are able to alter intracellular ion concentrations are attractive as novel anticancer agents. In particular, small molecule-based anion transporters have received particular attention due to their potential as therapeutic agents. We have for the first time shown that synthetic chloride ion transporters promote apoptosis by inducing sodium chloride influx, an increased level of reactive oxygen species, the release of cytochrome c from the mitochondria and caspase activation. Later, we have also found that a squaramide-based chloride ion transporter disrupts autophagy and induces apoptosis by perturbing cellular chloride concentrations. These results were published in Nature Chemistry in 2014 and 2017 respectively. We have also shown that synthetic glycan receptors promote apoptosis through caspase activation by binding to cell surface mannosides. Based on such findings, we are discovering biofunctional supramolecules that can be used as therapeutic agents. |
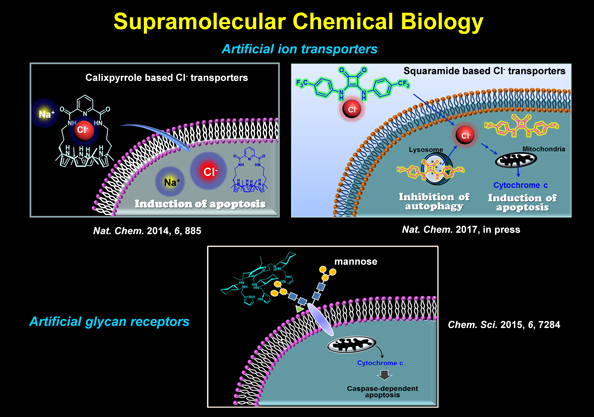 |
|

|
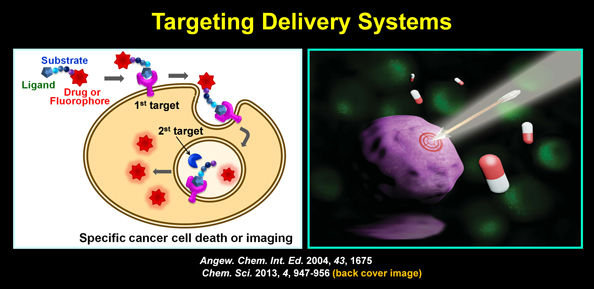
| Every year, a number of new anticancer agents are developed over the world. However, the discovery of safe and efficacious anticancer drugs is a highly difficult task owing mainly to their lack of selectivity, which causes unwanted normal cell death (i.e., toxic side effects). To minimize toxic side effects of anticancer agents, elegant methods that more specifically target cancer cells are required in order to improve chemotherapy efficacy. In this regard, we have developed novel drug delivery systems that specifically target disease cells. For example, we have devised dual-targeting delivery systems that specifically kill cancer by targeting receptors that are overexpressed in cancer as well as intracellular enzymes that are also overexpressed in cancers. We found that this delivery system more selectively kills cancer than does the single-targeting delivery system. |
 |
|
|
|
